
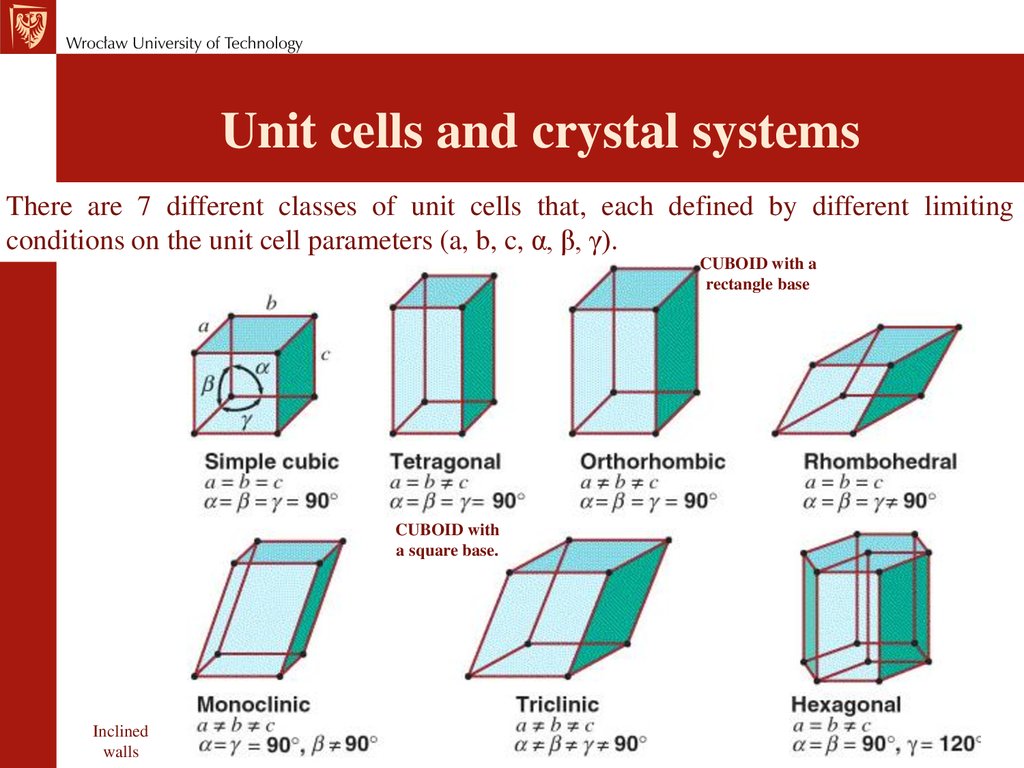
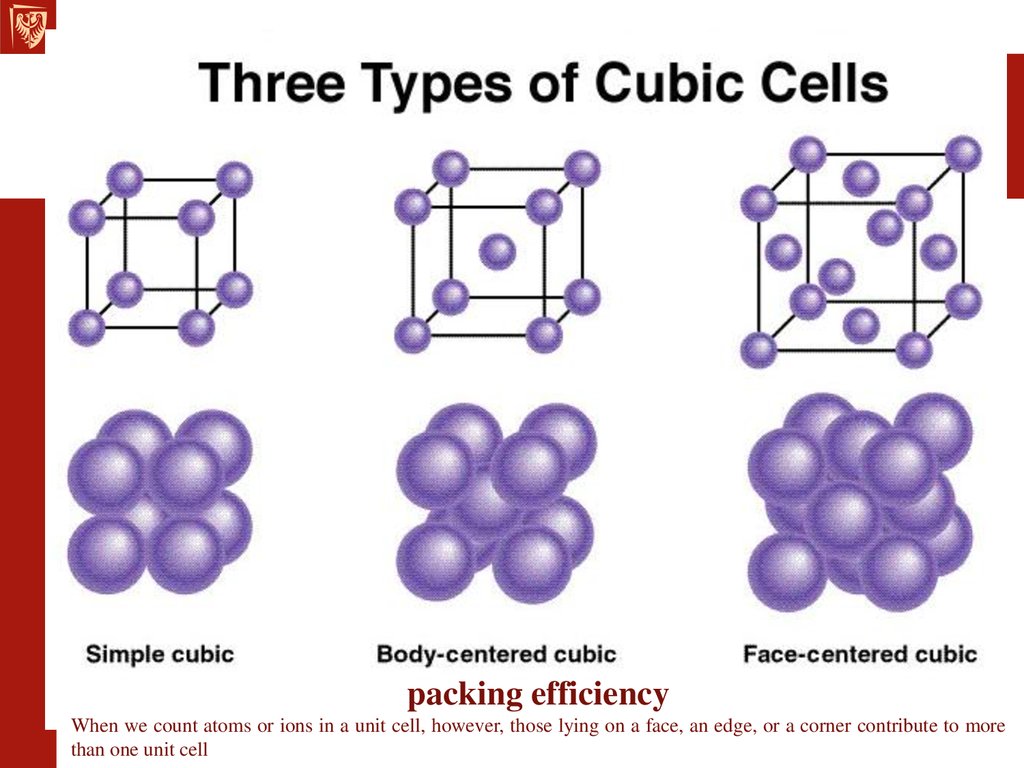
Determine which is larger when comparing a neutral atom to an anion/cation.Ionic compounds consist of regular repeating arrays of alternating cations and anions. (Assume the ionization states shown in Figure 6.14.) Covalent radii are in parentheses.
Learning Strategies Is lil molecular or ionic Ionic radii. This group contains non-metals and metalloids. Create a table showing the atomic and ionic radii of carbon, nitrogen, and oxygen. It is calculated from the internuclear distance between a cation and a neighboring anion in a lattice. L., a pioneer in ozone research) Raji Heyrovska* Na Stahlavce 6, 16000 Praha 6, Czech Republic, Email: "Geometry has two great treasures: one is the theorem of Pythagoras, the other the division of a line into mean and extreme ratio. and Golden ratio based ionic radii of oxygen (Dedicated to Mario J. However, since the additivity of a given set of radii was not found applicable to all bond types, sepa- rate tables of radii were derived for ionic, covalent, and metallic bonding. Ionic radii are typically reported in picometers (pm, 1 × 10 Ionic radii are typically given in units of either picometers (pm) or Angstroms (Å), with 1 Å = 100 pm. Trends in ionic radii XÄ NaX AgX F 464 492 Cl 564 555 Br 598 577 Unit cell parameters (in pm, equal to two MÄX bond lengths) for sodium and silver halides. The ionic radius is similar to but different from the atomic radius for the ionic size is dependent on the distribution of its outermost electrons and is inversely proportional to the effective nuclear charge experienced by ions. The ionic radii of metallic elements is smaller than its atomic radii, because the ion has less electrons.
The ionic radii (in Å) of N3–, O2– and F– are respectively Periodic Properties The ionic radii (in Å) of N 3–, O 2– and F – are respectively. Click hereto get an answer to your question ️ The ionic radii of O^2 -, F^ -, Na^ + and Mg^2 + are 1.35, 1.34, 0.95 and 0.66 A respectively. reference-request periodic-trends atomic-radius. However, these sizes can also be expressed in terms of van der Waals radii, determined empirically from the equilib rium internuclear distances between atoms which are in contact but not chemically bonded. Ionic radii (in a crystal lattice) - The measure of half the distance between two ions that are barely in contact.

Typical values range from 30 pm (0.3 Å) to over 200 pm (2 Å).


 0 kommentar(er)
0 kommentar(er)
